Strengthening a Tiny Heart, Pound for Pound
Says Dr. Bernhard Kühn, “If we can give these kids just half an ounce [more] heart muscle, that may potentially make a big difference for the duration and quality of their lives.”
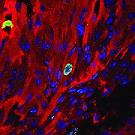
As reported by Heather Boerner of PittMed, the heart of a newborn is small but powerful—usually. For babies born with heart defects, though, the muscle can be marred by holes and malformed valves, or simply pump weakly. Surgery can close the holes and fix the valves.
But a weak heart? Medicine hasn’t been able to do much about that. Beta blockers and ACE inhibitors, common treatments for adults, are ineffective in children. Worse, corrective surgeries can cause scar tissue that further limits the heart’s power to pump. Left untreated, a weak heart can cause pediatric heart failure.
“This is where regeneration comes in,” says McGowan Institute for Regenerative Medicine affiliated faculty member Bernhard Kühn, an MD associate professor of pediatrics at Pitt and director of research in the Division of Pediatric Cardiology at Children’s Hospital of Pittsburgh of UPMC. “If we can give these kids just half an ounce [more] heart muscle, that may potentially make a big difference for the duration and quality of their lives.”
And it seems Dr. Kühn might have found a way to do that, at least in the lab. As reported in a Science Translational Medicine paper published in April, Dr. Kühn’s team applied to injured mouse hearts and isolated human tissue a protein called recombinant growth factor neuregulin-1 (rNRG1). The protein is known to stimulate cell growth in many organs and the nervous system. Dr. Kühn’s team stimulated cell growth. This complex, multipart study not only found rNRG1 to be effective but also pinpointed the narrow window in which to target future clinical trials.
In a previous study, Dr. Kühn and his team found that people with healthy hearts can generate new heart cells until about age 20. But no one knew how long an infant with an unhealthy heart would be able to grow new heart cells. So they set out to answer that question, as well as the question of whether rNRG1 could kick-start the process.
After fine-tuning their own adaptation of an animal model of pediatric heart disease, the team studied the timing of rNRG1 administration. They had assumed starting at 4 days old would be plenty early—but not so. Frustrated, they started all over again, introducing the protein at birth.
Everything changed. “When we started seeing positive results,” says Balakrishnan Ganapathy, a lab manager and research technologist at Children’s, “we realized we were on to something really big.”
Among rodents that received rNRG1 at birth, only one in 10 still had a scar across the entire thickness of the heart wall when they were examined later (compared to 75 percent of the hearts that received rNRG1 at 4 days old). That is to say, rNRG1 seems to generate cell growth, which can repair heart damage and build a stronger muscle. What’s more, rNRG1 seems to protect healthy heart tissue from dying.
“We do now have, in the data, some indication that the scar is now pumping [blood],” says Dr. Kühn.
At the same time, the lab was studying human heart tissue from infants who’d undergone heart surgery. They found that in the dish, the heart cells stopped proliferating in patients older than 6 months of age. And for those infants who did exhibit cell growth, it was slow.
But when they applied rNRG1 to the cells? “The surprising thing is that the heart-muscle pieces actually liked it,” says Dr. Kühn. “They had striations, so their molecular motors were visible with the microscope. The striations were still parallel, meaning when they contracted, they all pulled in synchronicity. The electrical connections were still present.”
Dr. Kühn and his team are hopeful that the same treatment might be effective in clinical trials that target children from birth to 6 months of age. Mr. Ganapathy, meanwhile, has begun studying the safety of rNRG1 administration in rodents. So far, he’s seen no serious side effects.
In this era of ever-improving neonatal medicine and surgery, the question is less often, Can we save this baby? says Dr. Kühn. “The question is,” he says, “How will this person live when he or she is 10, 20, 30, 40, 50, 60, 70, even 80 years old?”
Illustration: A Pitt lab may have found a way to strengthen and heal cardiac tissue in infants born with weak hearts. Here we see a cardiomyocyte (red) undergoing cellular proliferation (green). Blue spots are cell nuclei. --Balakrishnan Ganapathy.
Read more…
An Ounce of Regeneration--How to strengthen a tiny heart, pound for pound
Pitt Med (Summer 2015)
Bio: Dr. Bernhard Kühn
Abstract (Science Translational Medicine; 2015 Apr 1;7(281):281ra45.)
But a weak heart? Medicine hasn’t been able to do much about that. Beta blockers and ACE inhibitors, common treatments for adults, are ineffective in children. Worse, corrective surgeries can cause scar tissue that further limits the heart’s power to pump. Left untreated, a weak heart can cause pediatric heart failure.
“This is where regeneration comes in,” says McGowan Institute for Regenerative Medicine affiliated faculty member Bernhard Kühn, an MD associate professor of pediatrics at Pitt and director of research in the Division of Pediatric Cardiology at Children’s Hospital of Pittsburgh of UPMC. “If we can give these kids just half an ounce [more] heart muscle, that may potentially make a big difference for the duration and quality of their lives.”
And it seems Dr. Kühn might have found a way to do that, at least in the lab. As reported in a Science Translational Medicine paper published in April, Dr. Kühn’s team applied to injured mouse hearts and isolated human tissue a protein called recombinant growth factor neuregulin-1 (rNRG1). The protein is known to stimulate cell growth in many organs and the nervous system. Dr. Kühn’s team stimulated cell growth. This complex, multipart study not only found rNRG1 to be effective but also pinpointed the narrow window in which to target future clinical trials.
In a previous study, Dr. Kühn and his team found that people with healthy hearts can generate new heart cells until about age 20. But no one knew how long an infant with an unhealthy heart would be able to grow new heart cells. So they set out to answer that question, as well as the question of whether rNRG1 could kick-start the process.
After fine-tuning their own adaptation of an animal model of pediatric heart disease, the team studied the timing of rNRG1 administration. They had assumed starting at 4 days old would be plenty early—but not so. Frustrated, they started all over again, introducing the protein at birth.
Everything changed. “When we started seeing positive results,” says Balakrishnan Ganapathy, a lab manager and research technologist at Children’s, “we realized we were on to something really big.”
Among rodents that received rNRG1 at birth, only one in 10 still had a scar across the entire thickness of the heart wall when they were examined later (compared to 75 percent of the hearts that received rNRG1 at 4 days old). That is to say, rNRG1 seems to generate cell growth, which can repair heart damage and build a stronger muscle. What’s more, rNRG1 seems to protect healthy heart tissue from dying.
“We do now have, in the data, some indication that the scar is now pumping [blood],” says Dr. Kühn.
At the same time, the lab was studying human heart tissue from infants who’d undergone heart surgery. They found that in the dish, the heart cells stopped proliferating in patients older than 6 months of age. And for those infants who did exhibit cell growth, it was slow.
But when they applied rNRG1 to the cells? “The surprising thing is that the heart-muscle pieces actually liked it,” says Dr. Kühn. “They had striations, so their molecular motors were visible with the microscope. The striations were still parallel, meaning when they contracted, they all pulled in synchronicity. The electrical connections were still present.”
Dr. Kühn and his team are hopeful that the same treatment might be effective in clinical trials that target children from birth to 6 months of age. Mr. Ganapathy, meanwhile, has begun studying the safety of rNRG1 administration in rodents. So far, he’s seen no serious side effects.
In this era of ever-improving neonatal medicine and surgery, the question is less often, Can we save this baby? says Dr. Kühn. “The question is,” he says, “How will this person live when he or she is 10, 20, 30, 40, 50, 60, 70, even 80 years old?”
Illustration: A Pitt lab may have found a way to strengthen and heal cardiac tissue in infants born with weak hearts. Here we see a cardiomyocyte (red) undergoing cellular proliferation (green). Blue spots are cell nuclei. --Balakrishnan Ganapathy.
Read more…
An Ounce of Regeneration--How to strengthen a tiny heart, pound for pound
Pitt Med (Summer 2015)
Bio: Dr. Bernhard Kühn
Abstract (Science Translational Medicine; 2015 Apr 1;7(281):281ra45.)
